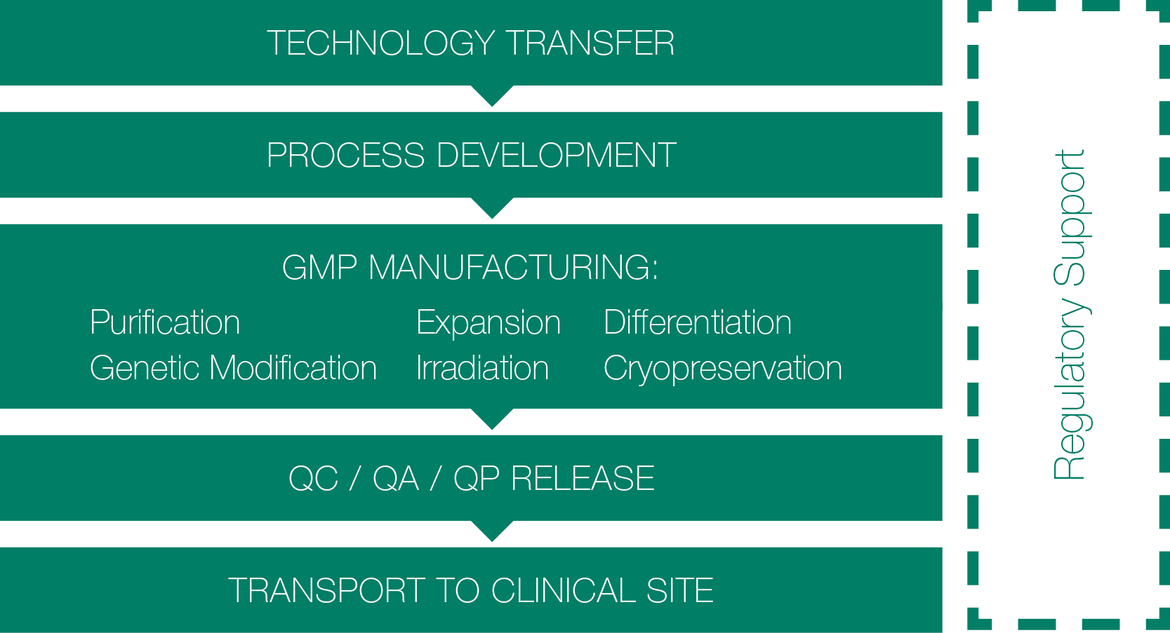
Independent of the type of cell product, autologous or allogeneic, native or gene modified or the respective medical indication, the manufacturing of innovative cell-based therapeutics is highly complex. BioNTech IMFS is an industry leader in the development and manufacturing services for cell-based therapeutics. Our commitment is to provide the highest quality service to support the rapid clinical development and commercial manufacturing of our customers’ products.
Our experienced process development team partners with our clients to develop specific custom protocols or to assure a timely and efficient tech-transfer of processes and QC assays. We are able to manufacture many primary cell types, as well as cell lines. We are specialists in the genetic modification of cells by different means and we have the capability to irradiate cells under GMP.
We have experience in handling a wide variety of cell types in systems that span from T-75 flasks to large cell culture bags/bioreactors. We meet your needs, encompassing every aspect of the process from raw material requirements, process flow, scalability, closed systems, fill & finish, Quality Control to storage and distribution.
A comprehensive QC and QA program ensures a robust manufacturing process and an exceptional and consistent batch-to-batch quality as well as compliance with regulatory requirements.
With a proven track record, BioNTech IMFS covers the full range of manufacturing services for cellular therapies. Starting from tech transfer and process development over clinical trial support to market supply, always embedded in a comprehensive quality management system.
Business Development
Dr. Andrea Schilz
Tel +49 (0) 6781 98550
andrea.schilz(at)biontech.de